Updates are crafted by Katie Havranek PhD, Vik Ghai PhD, Chris Booth PhD, David Booth, Claire Seibold, Tre Blohm, and other team members at FYR Diagnostics.
Contents:
- FYR Pandemic Response Team Updates – Archive
- Omicron Summary
- Updates on PCR and rapid antigen testing for SARS-CoV-2
- Are any new variants emerging? When does a variant become a variant of concern (VOC)?
- Updates about variant-specific vaccinations
- References
- Contact FYR
FYR Pandemic Response Team Updates:
Omicron Summary
The World Health Organization (WHO) designated the Omicron variant, Pango lineage B.1.1.529, as a Variant of Concern (VOC) on November 26, 2021. The first confirmed Omicron case in the U.S. was identified Dec. 1, 2021. Omicron contains 45-52 amino acid changes, with more than half of these changes occurring in the viral S-gene region that codes for the spike protein. The spike protein mediates viral entry into host cells and is the primary immunogen used in COVID-19 vaccines to induce protection against SARS-CoV-2 infection. The substantial number of mutations in Omicron has altered the host-pathogen interface, leading to changes in transmissibility, pathogenicity, and vaccine effectiveness.
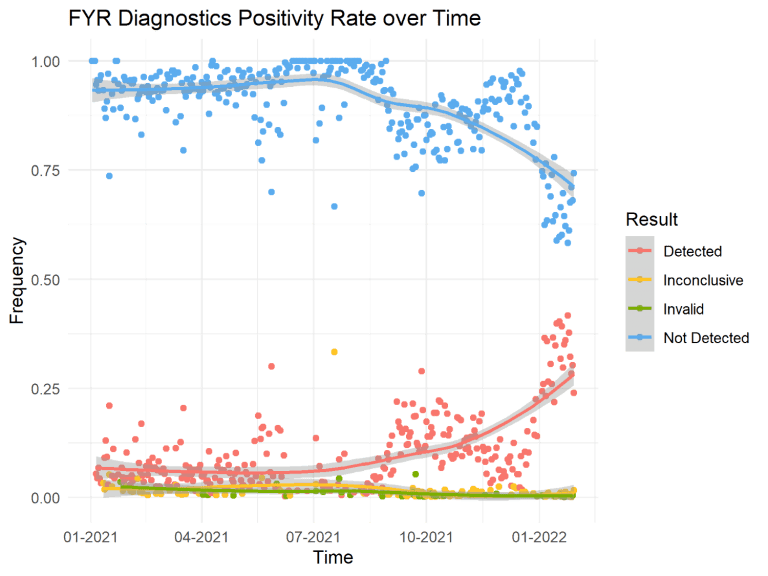
Since FYR’s last January 7, 2022 update, the Omicron (B.1.1.529) variant accounts for 99.9% of COVID-19 cases in the U.S., according to the CDC’s Nowcast variant proportion prediction model. The Delta variant has been nearly completely overtaken by Omicron, with Delta cases accounting for less than 1% of infections across the country. Internal sequencing at FYR has yielded similar percentages for the month of January, with just slightly over 1% of cases classified as a Delta lineage, and the other 99% Omicron. The 7-day moving averages for reported COVID-19 cases in the U.S. saw a drop of 5.0% last week, although we have experienced a record-breaking peak in COVID-19 cases in January that has been unlike any previous wave. The New York Times writes on January 31, 2022, “Even as the national data improves, a few states continue to see spikes in new cases. In Montana, infections have reached record levels and hospitalizations continue to rise.” FYR Diagnostic’s positivity rate since January 2021 are shown below in Figure 1. The unique molecular characteristics of Omicron are responsible for the unprecedented levels of community transmission we are currently experiencing.
FYR maintains close contact with state and federal officials and actively collaborates with the Montana Department of Public Health and Human Services. To combat the spread of COVID-19, FYR recommends use of PPE and following the direction of the CDC and other government authorities.
Updates on PCR and rapid antigen testing for SARS-CoV-2
The FDA has provided an online resource listing tests for which performance may be impacted by viral mutations (source below).
It has become increasingly clear that the emergence of novel variants will continue to drive the COVID-19 pandemic. The FDA has recently updated its overview lists of approved molecular diagnostics tests and antigen diagnostic tests for SARS-CoV-2 to make some important information easily accessible, including whether a test detects one viral gene or protein target, or multiple targets. The FDA states that single target tests are ‘more susceptible to changes in performance due to viral mutations, meaning they are more likely to fail to detect new variants’ and multi-target tests are ‘more likely to continue to perform well when new variants emerge’. FYR’s RT-PCR molecular diagnostic test is built to detect three different viral targets in the SARS-CoV-2 genome. Unfortunately, nearly all currently approved rapid antigen tests detect only a single viral target, which means that they are more likely to fail or exhibit decreased sensitivity when faced with a heavily mutated viral variant.
As discussed in our previous update, preliminary studies indicate that some antigen tests display reduced ability to detect the Omicron variant. At this point there have been multiple reports and studies showing that while antigen tests can still detect Omicron during the peak phase of infection when the viral load is highest, antigen tests are not reliable during the early days of infection, even if an individual is infectious. A person will typically test positive with a PCR test multiple days prior to testing positive with an antigen test, which leads to an increase in false negatives from antigen tests during the early stages of infection.
When does a variant become a variant of concern? Are any new variants emerging?
A variant of concern (VOC) is classified by the WHO when it surpasses variant of interest (VOI) status based on changes that may significantly impact global public health. A VOI exhibits genetic changes that are predicted to affect important viral characteristics (transmissibility, severity, immune escape, diagnostic escape, therapeutic escape) AND has demonstrated community transmission. A VOI is upgraded to a VOC when it displays, at a level of global public health significance, enhanced transmissibility or virulence, and/or a decrease in effectiveness of diagnostics, vaccines, therapeutics, and social/public health measures.
The FYR team continues to use advanced next generation sequencing (NGS) technology to survey 10-15% of confirmed COVID-positive samples for emergence of novel variants. If a novel VOC is classified by the WHO of CDC, FYR can quickly adapt its PCR-based variant screening methodology to identify an emerging VOC for sequencing. This is important because not all positive samples can be sequenced, but pre-screening enables the team to prioritize novel variants for sequencing.
The Omicron variant (B.1.1.529) encompasses the BA.1 (B.1.1.529.1), BA.2 (B.1.1.529.2) and BA.3 (BA.1.1.529.3) sublineages. BA.1 currently dominates locally and internationally, but recent data from several countries like Denmark, the United Kingdom, India, and South Africa show that BA.2 is increasing in proportion. While BA.2 has many of the same mutations as BA.1, BA.2 has even more mutations when compared with the original SARS-CoV-2 genome. BA.2 has also been harder to identify, thus its nickname of ‘stealth omicron’, because it does not have the mutation that causes S gene target failure, or SGTF, which was introduced in our Dec. 17 update. This does not mean that molecular diagnostics tests cannot detect BA.2, but rather that a BA.2 positive PCR test might look just like a Delta positive test, with neither one exhibiting SGTF. Many laboratories rely on SGTF during COVID-19 testing to identify presumptive Omicron cases because BA.1 and BA.3 have the mutation that causes SGTF. This may have allowed BA.2 to fly under the radar of detection since it may not get prioritized for sequencing as often as BA.1.
BA.2 has been identified in several states in the U.S., including Washington. Early reports from Denmark, where BA.2 accounts for nearly 50% of COVID-19 cases, indicate that the risk of hospitalization after infection with BA.2 appears to be roughly the same as with BA.1. At this time, it is unclear how the additional mutations in BA.2 will impact its spread and pathogenicity, although preliminary reports seem to show that BA.2 is roughly 1.5x more transmissible than BA.1. BA.2 is currently classified as a sublineage of Omicron, so it is currently not a VOC or VOI that is distinct from the other Omicron lineages. However, the WHO posted an update last week that calls for investigations into the unique characteristics of BA.2 as well as studies comparing BA.1 and BA.2. If BA.2 demonstrates significant differences from BA.1 from a global health perspective, the variant may receive its own VOC status distinct from BA.1.
Here is a public COVID-19 variant tracker that lists variants by their transmissibility, disease severity, vaccine effectiveness, and original county of origin: https://www.axios.com/variants-tracker.
Updates about variant-specific vaccinations
Vaccination and prior infection offer some level of protection against Omicron, but Omicron breakthrough infections and reinfections are more probable than with previous variants. Scientists from Pfizer/BioNTech have published an article that highlights the importance of a booster dose for protection against Omicron. Despite their decreased effectiveness against Omicron, existing vaccines have been shown to protect against the worst outcomes of SARS-CoV-2 infection, even for the Omicron variant. Both Pfizer and Moderna are in the process of conducting clinical trials for Omicron-specific mRNA technology-based vaccine booster doses.
FYR defers to the CDC and WHO for guidance related to vaccines.
References
SARS-CoV-2 Viral Mutations: Impact on COVID-19 Tests. https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/sars-cov-2-viral-mutations-impact-covid-19-tests#omicron.
WHO Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/sars-cov-2-viral-mutations-impact-covid-19-tests#omicron variant-ofconcern.
CDC COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/#variant-proportions.
New York Times Covid in the U.S: Latest Map and Case Count. https://www.nytimes.com/interactive/2021/us/covid-cases.html
FDA: In Vitro Diagnostics EUAs – Molecular Diagnostic Tests for SARS-CoV-2. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-molecular-diagnostic-tests-sars-cov-2.
FDA: In Vitro Diagnostics EUAs – Antigen Diagnostic Tests for SARS-CoV-2. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-antigen-diagnostic-tests-sars-cov-2.
Analytical sensitivity of seven SARS-CoV-2 antigen-detecting rapid tests for Omicron variant. https://www.medrxiv.org/content/10.1101/2021.12.18.21268018v1.full-text.
SARS-CoV-2 Omicron strain exhibits potent capabilities for immune evasion and viral entrance. https://www.nature.com/articles/s41392-021-00852-5.
Updates to Omicron lineage B.1.1.529. https://www.pango.network/updates-to-omicron-lineage-b-1-1-529/.
Pfizer and BioNTech Initiate Study to Evaluate Omicron-Based COVID-19 Vaccine in Adults 18-55 Years of Age. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-initiate-study-evaluate-omicron-based.
Moderna Announces First Participant Dosed in Phase 2 Study of Omicron-Specific Booster Candidate and Publication of Data on Booster Durability Against Omicron Variant. https://investors.modernatx.com/news/news-details/2022/Moderna-Announces-First-Participant-Dosed-in-Phase-2-Study-of-Omicron-Specific-Booster-Candidate-and-Publication-of-Data-on-Booster-Durability-Against-Omicron-Variant/default.aspx.
Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. https://www.science.org/doi/10.1126/science.abn7591
UK Health Security Agency: Covid-19 vaccine surveillance report. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1050721/Vaccine-surveillance-report-week-4.pdf
Contact FYR
FYR Diagnostics Inc
1121 E Broadway St.
Missoula, MT 59802
COVID-19: Covid19@fyr.bio
Press: Press@fyr.bio
Background on FYR Diagnostics
FYR (pronounced “Fire”) Diagnostics is a Montana-based company focused on developing and commercializing novel technology for diagnostics and testing in human health, life sciences, and agriculture. FYR Diagnostics is currently developing diagnostic solutions for cancers, neurological disorders, agricultural diseases, and neonatal-associated syndromes.
Thanks to support from the State of Montana, FYR’s partnership with the State Lab has increased statewide COVID-19 testing capacity and reduced the time patients must wait to receive results. For more information, visit fyrdiagnostics.com.
Disclaimer
FYR Diagnostics does not make any representation or warranties with respect to the accuracy, applicability, fitness, or completeness of the above content and/or any associated materials, collectively (“Materials”). FYR Diagnostics assumes no responsibility for errors or omissions in the contents of the Materials. FYR Diagnostics hereby disclaims any and all liability to any party for any direct, indirect, implied, punitive, special, incidental, or other consequential damages arising directly or indirectly from any use of the Materials, which is provided as is, and without warranties. Please seek advice from your healthcare provider regarding your personal healthcare questions.
