
Updates are crafted by Chris Booth PhD, Katie Havranek PhD, Vik Ghai PhD, David Booth, Claire Seibold, Tre Blohm, and other team members at FYR Diagnostics.
Contents:
- FYR Pandemic Response Team Updates
- Summary
- Where is Omicron (B.1.1.529)? Has Omicron been detected in Montana?
- Updates about current PCR and antigen testing of Omicron
- Updates about vaccine efficacy against Omicron
FYR Pandemic Response Team
Summary
Since FYR’s last December 17, 2021 update, there have been various Omicron (B.1.1.529) developments, ranging from an enhanced understanding of this variant and its unique qualities to a better understanding of viral spread and severity of disease.
The World Health Organization (WHO) designated the Omicron variant, Pango lineage B.1.1.529, as a Variant of Concern (VOC) on November 26, 2021. Omicron contains 45-52 amino acid changes, with more than half of these changes occurring in the viral S-gene region that codes for the spike protein. The spike protein mediates viral entry into host cells and is the primary immunogen used in COVID-19 vaccines to induce protection against SARS-CoV-2 infection. Due to the unprecedented number of mutations in Omicron, there has been considerable concern and uncertainty regarding the impact of these changes on immune evasion, vaccine-mediated protection, transmissibility, and disease severity.
FYR maintains close contact with state and federal officials and actively collaborates with the Montana Department of Public Health and Human Services. To combat the spread of Omicron, FYR recommends use of PPE and following the direction of the CDC and other government authorities.
Where is Omicron (B.1.1.529)? Has Omicron been detected in Montana?
Since FYR’s December 17th update, Omicron has been detected in nearly all countries and across the United States. FYR Diagnostics sequenced the first case of Omicron in Missoula County and the third case in Montana.
The FYR team continues to use advanced next generation sequencing (NGS) technology to monitor variants of concern from confirmed COVID-positive samples and continues to sequence 10-15% of confirmed COVID-positive samples on a weekly basis. To more rapidly detect Omicron in positive COVID samples, FYR is using its PCR-based variant screening methodology to quickly identify potential Omicron samples to prioritize for sequencing. Using this method, we have been able to predict what samples may be Omicron and can prioritize these samples for sequencing. There are currently 136 confirmed cases of Omicron in Montana, of which FYR has sequenced ~20% of those samples. Interestingly, there is an excellent correlation between the percentage of samples FYR has predicted to be Omicron using its screening methodology and confirmed Omicron cases in Montana as determined by sequencing (Figure 1)
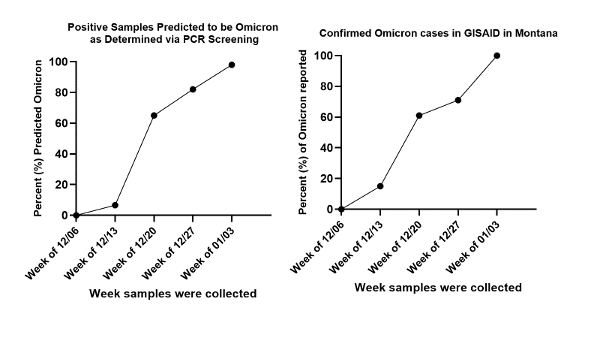
Figure 1: Comparison between positive samples predicted to be Omicron (left) and Confirmed cases in Montana during the initial Omicron outbreak (right).
Now that the vast majority of COVID-19 cases are shown to be caused by the Omicron variant in Montana, the screen will no longer be deployed, but will play a critical role at FYR in the future as new variants emerge and initial breakouts need to be tracked.
Updates about current PCR and antigen testing of Omicron
The FDA has provided an online resource listing tests for which performance may be impacted by viral mutations (source below), including an update on rapid antigen test sensitivity studies the FDA is directing in collaboration with the National Institutes of Health (NIH).
We are beginning to gain a better understanding of how rapid antigen detection tests are impacted by genetic changes in the Omicron variant of SARS-CoV-2. Rapid antigen tests vary widely in sensitivity, and it is well known that they are less sensitive overall than RT-PCR based diagnostic tests. However, they remain a convenient and widely used tool to rapidly diagnose symptomatic individuals. Most SARS-CoV-2 rapid antigen tests target the viral nucleocapsid (N) protein in order to avoid relying on the spike protein, which is more prone to change as new variants emerge. Although the N protein is less divergent than the spike protein, N still exhibits some differences between VOCs, which can impact diagnostic test performance.
Although clinical studies using patient samples with live virus are ongoing, preliminary laboratory tests from multiple sources indicate that antigen tests can detect the Omicron variant, but with reduced sensitivity. A preprint case study suggests that rapid tests are more likely to misdiagnose Omicron infection in its early stages, even though the infected individuals were able to spread the virus to others. However, RT-PCR based testing was able to detect Omicron infection in these individuals up to 3 days earlier, on average, than rapid antigen tests. This indicates that relying on rapid antigen tests for routine screening in asymptomatic or pre-symptomatic individuals may not be the best practice for controlling viral spread because false negative results may occur in the early phases of infection.
Updates about vaccine efficacy against Omicron
Laboratory and epidemiological data demonstrated that Omicron significantly escapes recognition by neutralizing antibodies conferred by prior infection and vaccination. Some early reports show that two doses of an mRNA vaccine series do not protect against Omicron infection, but a third dose provides around 37% effectiveness. This contrasts with vaccine effectiveness against the Delta VOC, which wanes over time after the first two doses, but is enhanced to 93% effectiveness after a third dose.
Importantly, Pfizer and BioNTech showed that while a two-dose course of the Pfizer-BioNTech COVID-19 Vaccine (BTN162b2) was less effective at antibody-mediated neutralization of Omicron when compared with other variants, most epitopes recognized by T-cells are still present in Omicron. The T-cell response is important for protection against severe disease and laboratory data also showed that vaccinated individuals have T-cell mediated immunity that shows cross-reactivity against Omicron and Delta VOCs. This means that vaccination and prior infection offer some level of protection against Omicron, but that Omicron breakthrough infections and reinfections are more probable than with previous variants. However, the severity of disease is likely to be mild due to robust T-cell mediated immunity. FYR defers to the CDC and WHO for guidance related to vaccines.
References
SARS-CoV-2 Viral Mutations: Impact on COVID-19 Tests. https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/sars-cov-2-viral-mutations-impact-covid-19-tests#omicron.
WHO Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. https://www.ecdc.europa.eu/en/publications-data/threat-assessment-brief-emergence-sars-cov-2-variant-b.1.1.529 variant-ofconcern.
European Centre for Disease Prevention and Control. Threat Assessment Brief: Implications of the emergence and spread of the SARS-CoV-2 B.1.1. 529 variant of concern (Omicron) for the EU/EEA. https://www.ecdc.europa.eu/en/publications-data/threat-assessment-brief-emergence-sars-cov-2-variant-b.1.1.529.
Analytical sensitivity of seven SARS-CoV-2 antigen-detecting rapid tests for Omicron variant. https://www.medrxiv.org/content/10.1101/2021.12.18.21268018v1.full-text.
SARS-CoV-2 Omicron strain exhibits potent capabilities for immune evasion and viral entrance. https://www.nature.com/articles/s41392-021-00852-5.
Reduced Neutralization of SARS-CoV-2 Omicron Variant by Vaccine Sera and monoclonal antibodies. https://www.medrxiv.org/content/10.1101/2021.12.07.21267432v2.full.pdf.
Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. https://www.medrxiv.org/content/10.1101/2021.11.11.21266068v2.full.pdf.
SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. https://www.medrxiv.org/content/10.1101/2021.12.08.21267417v1.full.pdf.
Effectiveness of COVID-19 vaccines against Omicron or Delta infection. https://www.medrxiv.org/content/10.1101/2021.12.30.21268565v1.full-text.
Vaccines Elicit High Cross-Reactive Cellular Immunity to the SARS-CoV-2 Omicron Variant. https://www.medrxiv.org/content/10.1101/2022.01.02.22268634v1.full.pdf
Updates to Omicron lineage B.1.1.529. https://www.pango.network/updates-to-omicron-lineage-b-1-1-529/.
Centers for Disease Control and Prevention COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/#variant-proportions.
Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8078597/.
Discordant SARS-CoV-2 PCR and Rapid Antigen Test Results When Infectious: A December 2021 Occupational Case Series. https://www.medrxiv.org/content/10.1101/2022.01.04.22268770v1.full.pdf+html.
Contact FYR
FYR Diagnostics Inc
1121 E Broadway St.
Missoula, MT 59802
COVID-19: Covid19@fyrdiagnostics.com
Press: Press@fyrdiagnostics.com
Background on FYR Diagnostics
FYR (pronounced “Fire”) Diagnostics is a Montana-based company focused on developing and commercializing novel technology for diagnostics and testing in human health, life sciences, and agriculture. FYR Diagnostics is currently developing diagnostic solutions for cancers, neurological disorders, agricultural diseases, and neonatal-associated syndromes.
Thanks to support from the State of Montana, FYR’s partnership with the State Lab has increased statewide COVID-19 testing capacity and reduced the time patients must wait to receive results. For more information, visit fyrdiagnostics.com.
Disclaimer
FYR Diagnostics does not make any representation or warranties with respect to the accuracy, applicability, fitness, or completeness of the above content and/or any associated materials, collectively (“Materials”). FYR Diagnostics assumes no responsibility for errors or omissions in the contents of the Materials. FYR Diagnostics hereby disclaims any and all liability to any party for any direct, indirect, implied, punitive, special, incidental, or other consequential damages arising directly or indirectly from any use of the Materials, which is provided as is, and without warranties. Please seek advice from your healthcare provider regarding your personal healthcare questions.
